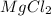Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
Determine the empirical formula of the compound formed when 1.2g of magnesium reacts with 3.55g of c...
Questions





Mathematics, 12.03.2020 21:25

Mathematics, 12.03.2020 21:25

Mathematics, 12.03.2020 21:26











Biology, 12.03.2020 21:26