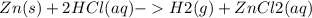A sample of brass weighing 1.203g was analyzed. The zinc in the alloy was reacted with 35.123g of HCl in excess, according to the balanced equation:
Zn (s) + 2HCl(aq) - > H2(g) + ZnCl2 (aq)
After all the zinc reacted, the mass of the remaining solution weighed 36.309g
What was the mass of H2 produced?
What mass of Zn reacted?
What was the percentage of Zn (by mass) in the alloy?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
A sample of brass weighing 1.203g was analyzed. The zinc in the alloy was reacted with 35.123g of HC...
Questions

Mathematics, 09.02.2021 14:00

Mathematics, 09.02.2021 14:00


Chemistry, 09.02.2021 14:00

English, 09.02.2021 14:00

Mathematics, 09.02.2021 14:00


Mathematics, 09.02.2021 14:00


Engineering, 09.02.2021 14:00


Mathematics, 09.02.2021 14:00


Medicine, 09.02.2021 14:00

Mathematics, 09.02.2021 14:00

Chemistry, 09.02.2021 14:00

Mathematics, 09.02.2021 14:00



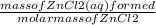
 = 0.0087 moles
= 0.0087 moles