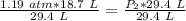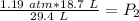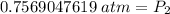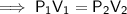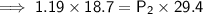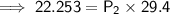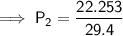Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1
You know the right answer?
A sample of oxygen gas at a pressure of 1.19 atm and a temperature of 24.4 °C, occupies a volume of...
Questions







History, 14.07.2021 01:00


Mathematics, 14.07.2021 01:00

English, 14.07.2021 01:00


English, 14.07.2021 01:00

Mathematics, 14.07.2021 01:00




Mathematics, 14.07.2021 01:10


Mathematics, 14.07.2021 01:10


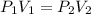
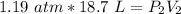

 . It is being multiplied by 29.4 liters. The inverse operation of multiplication is division. Divide both sides of the equation by 29.4 L.
. It is being multiplied by 29.4 liters. The inverse operation of multiplication is division. Divide both sides of the equation by 29.4 L.