
Chemistry, 12.08.2021 18:20 Jasminehenry123
Q1: A stock solution containing Mn+2 ions was prepared by dissolving 1.584 g pure manganese metal in nitric acid and diluting to a final volume of 1.000 L. The following solutions were then prepared by dilution: For solution A, 50.00 mL of stock solution was diluted to 1000.0 mL. For solution B, 10.00 mL of solution A was diluted to 250.0 mL. For solution C, 10.00 mL of solution B was diluted to 500.0 mL. Calculate the concentrations of the stock solution and solutions A, B, and C.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
Q1: A stock solution containing Mn+2 ions was prepared by dissolving 1.584 g pure manganese metal in...
Questions


Chemistry, 18.04.2020 07:32

Mathematics, 18.04.2020 07:32

Mathematics, 18.04.2020 07:32







Mathematics, 18.04.2020 07:33


Mathematics, 18.04.2020 07:33


Biology, 18.04.2020 07:33




Physics, 18.04.2020 07:34

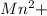 = 1.584 g/55g/mol = 0.0288 moles
= 1.584 g/55g/mol = 0.0288 moles

