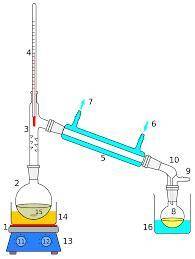
PLEASE HELP The boiling point of water is 100ºC. The boiling point of acetone is 56ºC. Which statement about distilling a mixture of acetone and water is correct?\
A. Water will vaporize from the mixture before acetone.
B. Water is collected as it leaves the mixture.
C. Acetone remains in the original container.
D. Acetone is captured and cooled.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1


Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
PLEASE HELP The boiling point of water is 100ºC. The boiling point of acetone is 56ºC. Which stateme...
Questions




Computers and Technology, 23.08.2019 14:30

Biology, 23.08.2019 14:30

English, 23.08.2019 14:30


Health, 23.08.2019 14:30

Mathematics, 23.08.2019 14:50


Social Studies, 23.08.2019 14:50


Computers and Technology, 23.08.2019 14:50


Mathematics, 23.08.2019 14:50

Mathematics, 23.08.2019 14:50