
Chemistry, 07.08.2021 03:00 korireidkdotdot5973
The half-reaction at the cathode in an electrochemical cell is given below.
What other half-reaction would most likely occur at the anode to produce a
spontaneous reaction?
Zn2+(aq) + 2e —> Zn(s)
A. H2(g) —> 2H+(aq) + 2e-
B. Al(s) —> Al3+ (aq) + 3e-
C. K^+ (aq) + e^- —> K (s)
D. Zn (s) —> Zn2^+ (aq) + 2e-
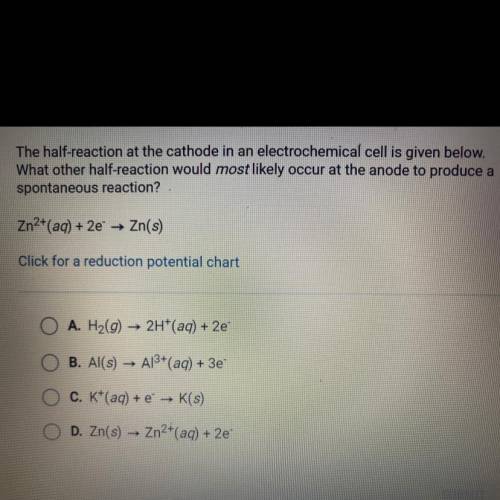

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3
You know the right answer?
The half-reaction at the cathode in an electrochemical cell is given below.
What other half-reactio...
Questions

English, 20.10.2021 02:20



Mathematics, 20.10.2021 02:20

History, 20.10.2021 02:20

Physics, 20.10.2021 02:20

History, 20.10.2021 02:20


Social Studies, 20.10.2021 02:20


Mathematics, 20.10.2021 02:30

Mathematics, 20.10.2021 02:30



English, 20.10.2021 02:30

Mathematics, 20.10.2021 02:30

History, 20.10.2021 02:30

Social Studies, 20.10.2021 02:30

Biology, 20.10.2021 02:30



