
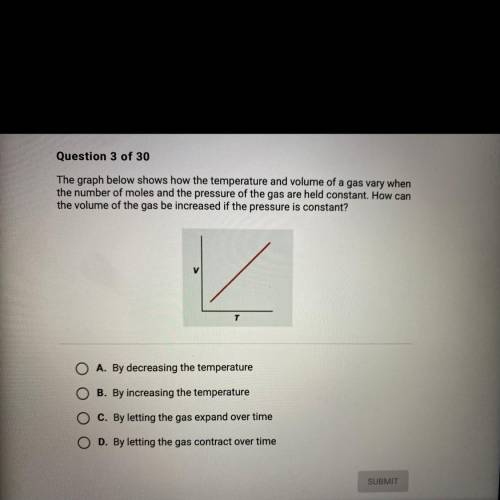
The graph below shows how the temperature and volume of a gas vary when
the number of moles and the pressure of the gas are held constant. How can
the volume of the gas be increased if the pressure is constant?
A. By decreasing the temperature
B. By increasing the temperature
C. By letting the gas expand over time
D. By letting the gas contract over time

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1

Chemistry, 23.06.2019 19:30
The melting point of an ionic compound is likely to be blank and michael molecula the melting point of an ionic compound is likely to be a molecular compound
Answers: 3
You know the right answer?
The graph below shows how the temperature and volume of a gas vary when
the number of moles and the...
Questions

English, 05.05.2020 21:05

Mathematics, 05.05.2020 21:05

Mathematics, 05.05.2020 21:05

Mathematics, 05.05.2020 21:05

Mathematics, 05.05.2020 21:05

Chemistry, 05.05.2020 21:05


Health, 05.05.2020 21:05






Mathematics, 05.05.2020 21:05


Chemistry, 05.05.2020 21:05

Mathematics, 05.05.2020 21:05


Mathematics, 05.05.2020 21:05

Mathematics, 05.05.2020 21:05



