
Chemistry, 02.08.2021 19:30 salinasroel22
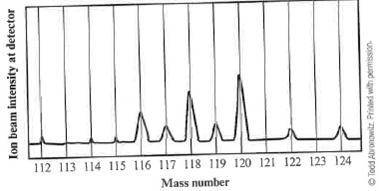
When a sample of an unknown element is vaporized and injected into a mass spectrometer, the results shown below are obtained. Use these data to estimate the average atomic mass of this element.
(A) 117 amu
(B) between 117 and 118 amu
(C) between 118 and 119 amu
(D) between 119 and 120 amu

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
When a sample of an unknown element is vaporized and injected into a mass spectrometer, the results...
Questions






Biology, 13.09.2019 21:10



Mathematics, 13.09.2019 21:10








Chemistry, 13.09.2019 21:10

Chemistry, 13.09.2019 21:10

Chemistry, 13.09.2019 21:10



