
Chemistry, 02.08.2021 17:10 andreamarie2004amg
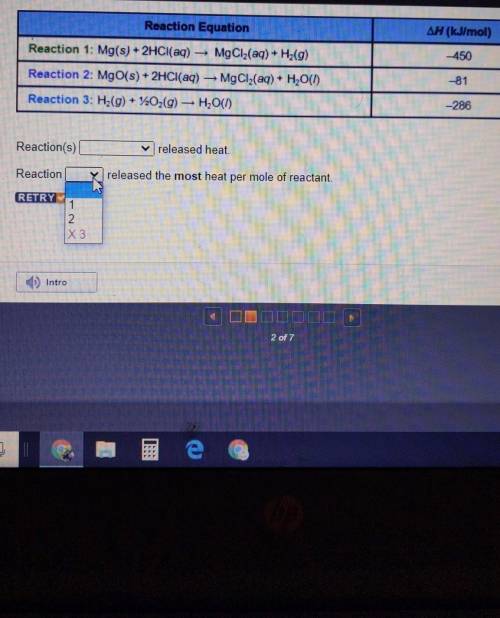
Reaction Equation ∆H (kJ/mol) -450 Reaction 1: Mg(s) + 2HCl(aq) — MgCl2(aq) + Hz(9) Reaction 2: MgO(s) + 2HCl(aq) — MgCl2(aq) + H2O(1) Reaction 3: H2(g) + 220,(9) — H2O(1) -81 -286
Reaction(s)_released heat. W
Reaction_released the most heat per mole of reactant

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
Reaction Equation ∆H (kJ/mol) -450 Reaction 1: Mg(s) + 2HCl(aq) — MgCl2(aq) + Hz(9) Reaction 2: MgO(...
Questions



Computers and Technology, 18.03.2021 03:00

Social Studies, 18.03.2021 03:00


Chemistry, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00


Biology, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00




Mathematics, 18.03.2021 03:00

Business, 18.03.2021 03:00


SAT, 18.03.2021 03:00



Mathematics, 18.03.2021 03:00



