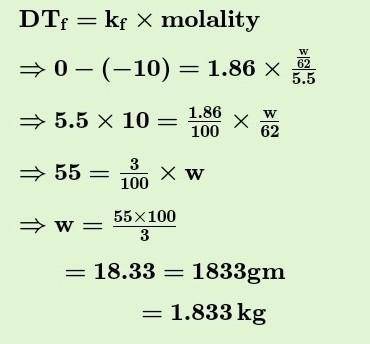
Chemistry, 02.08.2021 04:40 anthonyjackson12aj
in order to decrease the freezing point of 500. g of water to 1.00° c how many grams of ethylene glycol (C2H602)must be added (KF=1.86°C kg solvent)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1


You know the right answer?
in order to decrease the freezing point of 500. g of water to 1.00° c how many grams of ethylene gly...
Questions


Mathematics, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01

Health, 05.10.2020 16:01


Chemistry, 05.10.2020 16:01




English, 05.10.2020 16:01

Social Studies, 05.10.2020 16:01

English, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01




Mathematics, 05.10.2020 16:01


Mathematics, 05.10.2020 16:01