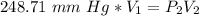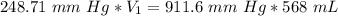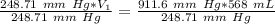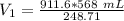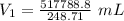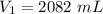Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1
You know the right answer?
A sample of oxygen occupied 568 ml. when the pressure increased to 911.6 mm Hg. at constant temperat...
Questions

English, 10.07.2021 16:20


Mathematics, 10.07.2021 16:20


Chemistry, 10.07.2021 16:20


Mathematics, 10.07.2021 16:20

World Languages, 10.07.2021 16:20

English, 10.07.2021 16:20



Mathematics, 10.07.2021 16:50

Geography, 10.07.2021 16:50




Chemistry, 10.07.2021 16:50


Mathematics, 10.07.2021 16:50

Mathematics, 10.07.2021 16:50