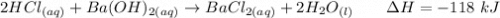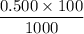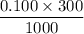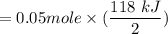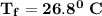Chemistry, 30.07.2021 23:00 maskythegamer
Calculate the heat when 100.0 mL of 0.500 M HCl is mixed with 300.0 mL of 0.100 M Ba(OH)2. Assuming that the temperature of both solutions was initially 25.0C and that the final mixture has a mass of 400.0 g and a specific heat capacity of 4.18 J/C g, calculate the final temperature of the mixture.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Calculate the heat when 100.0 mL of 0.500 M HCl is mixed with 300.0 mL of 0.100 M Ba(OH)2. Assuming...
Questions

Health, 18.10.2020 21:01

Mathematics, 18.10.2020 21:01

Mathematics, 18.10.2020 21:01

Geography, 18.10.2020 21:01

Mathematics, 18.10.2020 21:01

Arts, 18.10.2020 21:01

Mathematics, 18.10.2020 21:01


Mathematics, 18.10.2020 21:01


Mathematics, 18.10.2020 21:01





Spanish, 18.10.2020 21:01


Mathematics, 18.10.2020 21:01

Mathematics, 18.10.2020 21:01