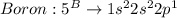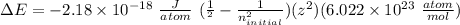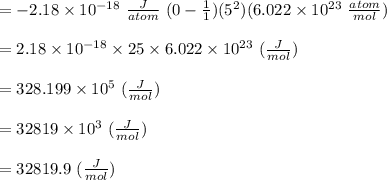Chemistry, 28.07.2021 21:00 xxheartbreakerxx11
Given the following formula for calculating the ionization energy of one-electron species such as Li2+, He+, and H, calculate the ionization energy (in J/mol) for B4+. Use scientific notation in answers (ex: 1E10, 3.20E-6)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
Given the following formula for calculating the ionization energy of one-electron species such as Li...
Questions

Mathematics, 09.09.2020 05:01

Computers and Technology, 09.09.2020 05:01

Business, 09.09.2020 05:01


Mathematics, 09.09.2020 05:01



English, 09.09.2020 05:01

Social Studies, 09.09.2020 05:01

English, 09.09.2020 05:01

English, 09.09.2020 05:01

Chemistry, 09.09.2020 05:01

Mathematics, 09.09.2020 05:01

English, 09.09.2020 05:01

English, 09.09.2020 05:01

Geography, 09.09.2020 05:01

Geography, 09.09.2020 05:01

Mathematics, 09.09.2020 05:01

Mathematics, 09.09.2020 05:01

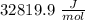 "
"