
Chemistry, 28.07.2021 03:30 MNBASKETBALL838
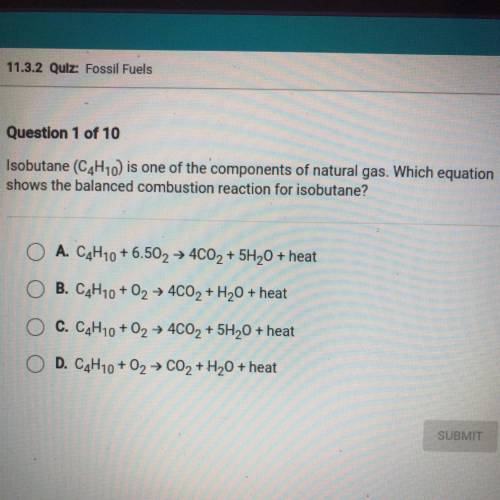
Isobutane (C4H10) is one of the components of natural gas. Which equation
shows the balanced combustion reaction for isobutane?
A. C4H10 +6.502 + 4CO2 + 5H20 + heat
B. C4H10 + O2 + 4C02 + H2O + heat
C. C4H10 + O2 + 4CO2 + 5H20 + heat
D. C4H10 + 02 → CO2 + H2O + heat

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3
You know the right answer?
Isobutane (C4H10) is one of the components of natural gas. Which equation
shows the balanced combus...
Questions



Mathematics, 31.01.2020 23:57


Mathematics, 31.01.2020 23:57


Biology, 31.01.2020 23:57


Mathematics, 31.01.2020 23:57


Mathematics, 31.01.2020 23:57




Mathematics, 31.01.2020 23:57


History, 31.01.2020 23:57


Social Studies, 31.01.2020 23:57




