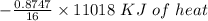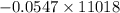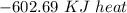Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
Using the following equation for the combustion of octane calculate the heat associated with the for...
Questions




Physics, 09.02.2021 16:20


Advanced Placement (AP), 09.02.2021 16:20








Mathematics, 09.02.2021 16:20




Mathematics, 09.02.2021 16:20


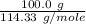
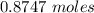
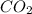 formation associates with -11018 kJ of heat, then
formation associates with -11018 kJ of heat, then