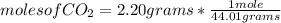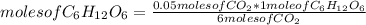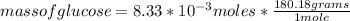Chemistry, 27.07.2021 14:00 joshvslogic341
The reaction for photosynthesis producing glucose sugar and oxygen gas is:
__CO2(g) + __H2O(l) UV/chlorophyl−→−−−−−−−−−−−−−− __C6H12O6(s) + __O2(g)
What is the mass of glucose (180.18 g/mol) produced from 2.20 g of CO2 (44.01 g/mol)?
a. 66.1 g C6H12O6
b. 396 g C6H12O6
c. 54.0 g C6H12O6
d. 1.50 g C6H12O6
e. 9.01 g C6H12O6

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Straightforward questions answered in the powerpoint slidesreaction: heating the starting materials under refluxwhat does it mean to heat under reflux? why do we choose water as the reflux solvent? what are boiling chips used for? why do we put a condenser on top of the reaction? why do we add heat and let the reaction stir for 30 minutes? why do we add sulfuric acid to the reaction after it cools as opposed to when it’s still hot? separation: filtration of precipitatewhy don’t we do an aqueous and organic extraction in the separatory funnel? why do you rinse the salicylic acid on the filter with ice cold water? purification: recrystallization of salicylic acid (no hot filtration needed)what is the difference in the amount of room temperature water vs. boiling water needed to dissolve the salicylic acid (assume a 1.2 gram yield of salicylic acid)? remember, in the lab if you need x ml of boiling water to dissolve a solid, then you should add a little more (definitely no more than 1.5 times the theoretical amount) to ensure it doesn’t recrystallize prematurely.analysis: melting point of salicylic acidwhat can you conclude if the melting point of the salicylic acid you just synthesized is 152-155oc and the 1: 1 mix of your product and “synthetic” salicylic acid is 151-154oc?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
The reaction for photosynthesis producing glucose sugar and oxygen gas is:
__CO2(g) + __H2O(l) UV/c...
Questions

Arts, 22.01.2021 14:00


Mathematics, 22.01.2021 14:00

Mathematics, 22.01.2021 14:00



Health, 22.01.2021 14:00




History, 22.01.2021 14:00


Mathematics, 22.01.2021 14:00

Business, 22.01.2021 14:00


English, 22.01.2021 14:00

History, 22.01.2021 14:00

Mathematics, 22.01.2021 14:00

History, 22.01.2021 14:00