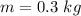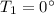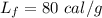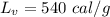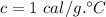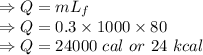Chemistry, 27.07.2021 04:40 theledfords855
Calculate the heat change in calories for melting of 0.30 kg of water at 0*C. The
heat of fusion for water is 80 cal/g. The heat of vaporization of water is 540 cal/g.
The specific heat capacity of water is 1.00 cal/g*C.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1
You know the right answer?
Calculate the heat change in calories for melting of 0.30 kg of water at 0*C. The
heat of fusion fo...
Questions




Mathematics, 19.05.2021 18:40

Mathematics, 19.05.2021 18:40



Health, 19.05.2021 18:40


Mathematics, 19.05.2021 18:40


Mathematics, 19.05.2021 18:40

Arts, 19.05.2021 18:40



Mathematics, 19.05.2021 18:40

Mathematics, 19.05.2021 18:40

Mathematics, 19.05.2021 18:40

Mathematics, 19.05.2021 18:40