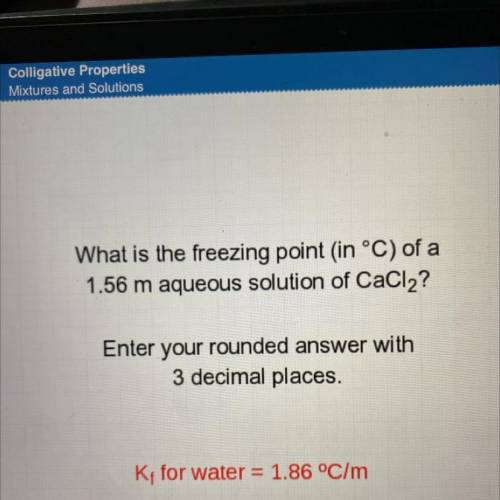
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded a...

Chemistry, 26.07.2021 22:20 joselinegarciaowyrpf
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded answer with
3 decimal places.
K; for water = 1.86 °C/m

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

You know the right answer?
Questions


Mathematics, 30.06.2020 01:01

Mathematics, 30.06.2020 01:01

Mathematics, 30.06.2020 01:01






Mathematics, 30.06.2020 01:01

Mathematics, 30.06.2020 01:01




Mathematics, 30.06.2020 01:01





Mathematics, 30.06.2020 01:01



