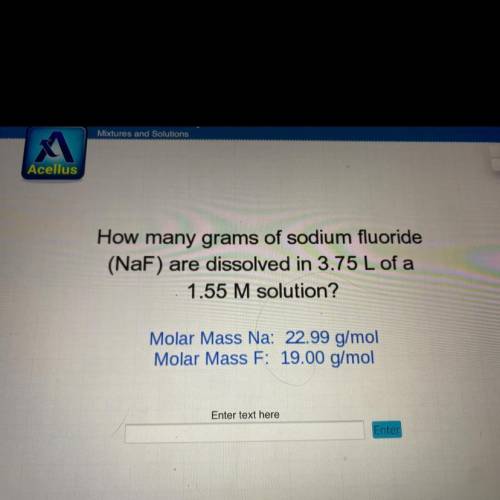
How many grams of sodium fluoride
(NaF) are dissolved in 3.75 L of a
1.55 M solution?
M...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

You know the right answer?
Questions


Advanced Placement (AP), 11.11.2020 21:40


Mathematics, 11.11.2020 21:40

English, 11.11.2020 21:40

Mathematics, 11.11.2020 21:40

English, 11.11.2020 21:40






Physics, 11.11.2020 21:40

Mathematics, 11.11.2020 21:40


Mathematics, 11.11.2020 21:40


Spanish, 11.11.2020 21:40

Mathematics, 11.11.2020 21:40