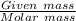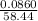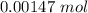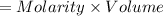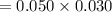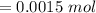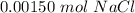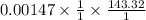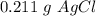Chemistry, 26.07.2021 17:40 matt416760
A sample of 0.0860 g of sodium chloride is added to 30.0 mL of 0.050 M silver nitrate, resulting in the formation of a precipitate. (a) Write the molecular equation for the reaction. (b) What is the limiting reactant in the reaction? (c) How many grams of precipitate potentially form?

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1
You know the right answer?
A sample of 0.0860 g of sodium chloride is added to 30.0 mL of 0.050 M silver nitrate, resulting in...
Questions

Mathematics, 12.01.2021 01:00


Mathematics, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00

Chemistry, 12.01.2021 01:00


Mathematics, 12.01.2021 01:00



Mathematics, 12.01.2021 01:00


Biology, 12.01.2021 01:00

Chemistry, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00

History, 12.01.2021 01:00


Chemistry, 12.01.2021 01:00


Mathematics, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00

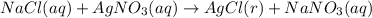
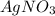 ,
,