
Chemistry, 24.07.2021 21:00 mickimlvn6128
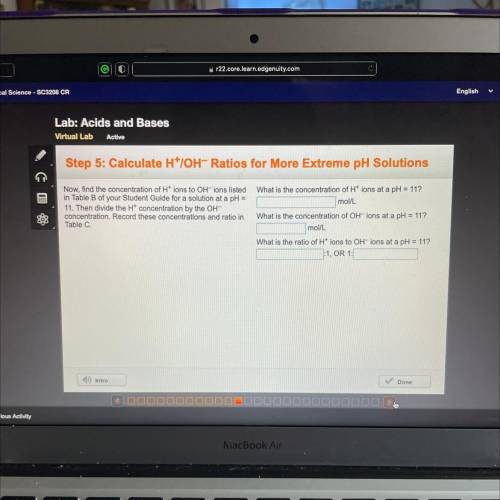
Step 5: Calculate H*IOH-Ratios for More Extreme pH Solutions
What is the concentration of H+ ions at a pH = 11?
mol/L
Now, find the concentration of H+ ions to OH-ions listed
in Table B of your Student Guide for a solution at a pH =
11. Then divide the Ht concentration by the OH-
concentration. Record these concentrations and ratio in
Table C.
What is the concentration of OH-ions at a pH = 11?
mol/L
What is the ratio of H+ ions to OH-ions at a pH = 11?
:1, OR 1:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
Step 5: Calculate H*IOH-Ratios for More Extreme pH Solutions
What is the concentration of H+ ions a...
Questions



Mathematics, 21.10.2019 22:30



Mathematics, 21.10.2019 22:30


Mathematics, 21.10.2019 22:30

Mathematics, 21.10.2019 22:30

English, 21.10.2019 22:30




Mathematics, 21.10.2019 22:30


Social Studies, 21.10.2019 22:30

Mathematics, 21.10.2019 22:30



Mathematics, 21.10.2019 22:30



