
Chemistry, 24.07.2021 06:40 steven2669
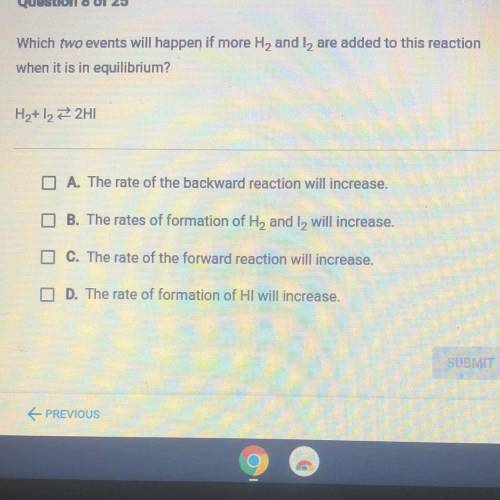
Which we events will happen if more H, and t, are added to this reaction
when it is in equilibrium?
A. The rate of the backward reaction will increase
The rates of formation of He and I will increase
0. The rate of the forward reaction will increase
D. The rate of formation of HI will increase

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

You know the right answer?
Which we events will happen if more H, and t, are added to this reaction
when it is in equilibrium?...
Questions

Mathematics, 04.05.2021 02:30

Mathematics, 04.05.2021 02:30



Mathematics, 04.05.2021 02:30


Arts, 04.05.2021 02:30

Mathematics, 04.05.2021 02:30



Mathematics, 04.05.2021 02:30



Mathematics, 04.05.2021 02:30

Mathematics, 04.05.2021 02:30



French, 04.05.2021 02:30




