
Chemistry, 21.07.2021 21:20 denisebaslee15
What direction would equilibrium moves towards based on the following if we increased the volume of the container.
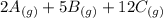 ↔
↔ 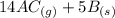
Answer choices:
a) reactants
b) no change
c) products
d) decrease in volume
Please help!

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1
You know the right answer?
What direction would equilibrium moves towards based on the following if we increased the volume of...
Questions


Business, 23.10.2021 20:10


Mathematics, 23.10.2021 20:10

History, 23.10.2021 20:10








English, 23.10.2021 20:10

SAT, 23.10.2021 20:10



Social Studies, 23.10.2021 20:10

English, 23.10.2021 20:10

English, 23.10.2021 20:10

Mathematics, 23.10.2021 20:10



