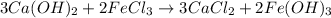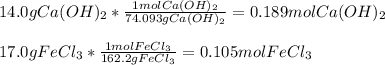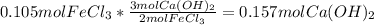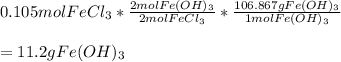Chemistry, 20.07.2021 04:10 kashmoney8690
A chemist dissolves 14.0 g of calcium hydroxide in one beaker of water, and 17.0 g of iron(III) chloride
in a second beaker of water. Everything dissolves.
When the two solutions are poured together, solid iron(III) hydroxide precipitates.
1. Write a balanced molecular equation.
2. Determine the identity of the limiting reactant.
3. Predict the mass of iron(III) hydroxide product.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
A chemist dissolves 14.0 g of calcium hydroxide in one beaker of water, and 17.0 g of iron(III) chlo...
Questions

Social Studies, 14.01.2021 17:10



Arts, 14.01.2021 17:10

Mathematics, 14.01.2021 17:10

Mathematics, 14.01.2021 17:10



Mathematics, 14.01.2021 17:10

Arts, 14.01.2021 17:10

Mathematics, 14.01.2021 17:10


Mathematics, 14.01.2021 17:10

History, 14.01.2021 17:10

Mathematics, 14.01.2021 17:10

English, 14.01.2021 17:10



Engineering, 14.01.2021 17:10

Mathematics, 14.01.2021 17:10