Chemistry, 19.07.2021 17:50 unicornpoop54
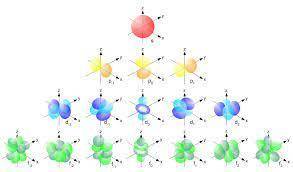
Which statement describes the 3d, 4s, and 4p orbitals of Arsenic (As) based on its electronic configuration and position in the periodic table?
The 3d and 4s orbitals are completely filled, and the 4p orbital is partially filled.
The 3d orbital is completely filled, and the 4s and 4p orbitals are partially filled.
The 3d, 4s, and 4p orbitals are completely filled.
The 3d, 4s, and 4p orbitals are partially filled.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3
You know the right answer?
Which statement describes the 3d, 4s, and 4p orbitals of Arsenic (As) based on its electronic config...
Questions

Geography, 18.04.2021 07:50



Mathematics, 18.04.2021 08:00


Biology, 18.04.2021 08:00

Mathematics, 18.04.2021 08:00





Physics, 18.04.2021 08:00




Mathematics, 18.04.2021 08:00


English, 18.04.2021 08:00