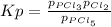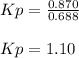A reaction vessel is charged with phosphorus pentachloride, which partially decomposes to phosphorus trichloride and molecular chlorine according to the following reaction:
PCl5(g)â PCl3(g)+Cl2(g)
When the system comes to equilibrium at 250.0°C, the equilibrium partial pressures are: PPCl5 = 0.688 atm and PPCl3 = PCl2 = 0.870 atm.
Required:
What is the value of Kp at this temperature?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
A reaction vessel is charged with phosphorus pentachloride, which partially decomposes to phosphorus...
Questions


Business, 11.02.2021 06:40

History, 11.02.2021 06:40

Mathematics, 11.02.2021 06:40

English, 11.02.2021 06:40




Health, 11.02.2021 06:40

Mathematics, 11.02.2021 06:40


Mathematics, 11.02.2021 06:40

World Languages, 11.02.2021 06:40

English, 11.02.2021 06:40

Advanced Placement (AP), 11.02.2021 06:40

Mathematics, 11.02.2021 06:40

English, 11.02.2021 06:40

Mathematics, 11.02.2021 06:40


Mathematics, 11.02.2021 06:40