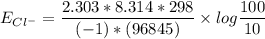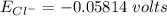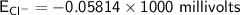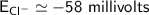The Nernst equation at 20oC is:
Eion= 58 millvolts/z. [log10 (ion)out/(ion)in]
Calculat...

Chemistry, 19.07.2021 17:10 shortty1111
The Nernst equation at 20oC is:
Eion= 58 millvolts/z. [log10 (ion)out/(ion)in]
Calculate the equilibrium potential for Cl- if the concentration of Cl- outside of the cell is 100 and the concentration inside of the cell is 10 mmol/liter.
a. 58 millivolts
b. +58 millivolts
c. -116 millivolts
d. 0

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
Questions

Biology, 24.02.2021 21:50


Mathematics, 24.02.2021 21:50

Mathematics, 24.02.2021 21:50


English, 24.02.2021 21:50


Biology, 24.02.2021 21:50

Mathematics, 24.02.2021 21:50




English, 24.02.2021 21:50


Chemistry, 24.02.2021 21:50

Mathematics, 24.02.2021 21:50

Chemistry, 24.02.2021 21:50



Mathematics, 24.02.2021 21:50

![E_{ion} = 58 millivolts /z \Big[ log_{10} \Big( \dfrac{[ion]_{out}}{[ion]_{in}}\Big) \Big]}](/tpl/images/1396/2001/4bd00.png)
![E_{Cl^-} = \dfrac{2.303*R*T}{ZF} \times log \dfrac{[Cl^-]_{out}} {[Cl^-]_{in}}](/tpl/images/1396/2001/9486b.png)