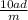Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1


Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
calculate molarity, molality and mole fraction of 28 percent aqueous solution of a solute with molar...
Questions

Mathematics, 26.02.2021 15:30





English, 26.02.2021 15:30


Mathematics, 26.02.2021 15:30

Physics, 26.02.2021 15:30

Physics, 26.02.2021 15:30

Mathematics, 26.02.2021 15:30



Mathematics, 26.02.2021 15:30

Mathematics, 26.02.2021 15:30

Biology, 26.02.2021 15:30


Mathematics, 26.02.2021 15:30