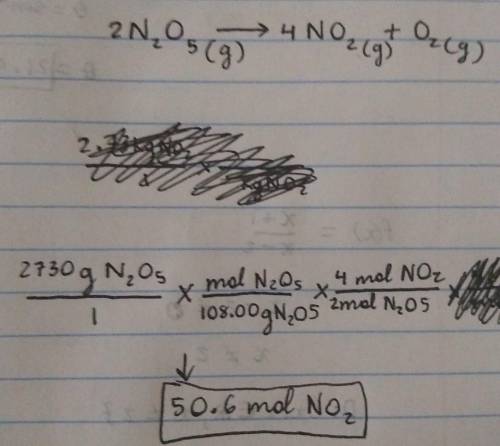Chemistry, 19.07.2021 03:40 anonymous1813
Calculate how many moles of NO2
form when each quantity of reactant completely reacts via the following reaction:
2N2O5(g)→4NO2(g)+O2(g)
2.73 kg N2O5
Express your answer using three significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1



Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3
You know the right answer?
Calculate how many moles of NO2
form when each quantity of reactant completely reacts via the follo...
Questions




Physics, 02.12.2019 21:31





Computers and Technology, 02.12.2019 21:31

Mathematics, 02.12.2019 21:31


Computers and Technology, 02.12.2019 21:31


History, 02.12.2019 21:31


Mathematics, 02.12.2019 21:31

Business, 02.12.2019 21:31


Mathematics, 02.12.2019 21:31

Mathematics, 02.12.2019 21:31