
Chemistry, 18.07.2021 01:40 joyandfaye
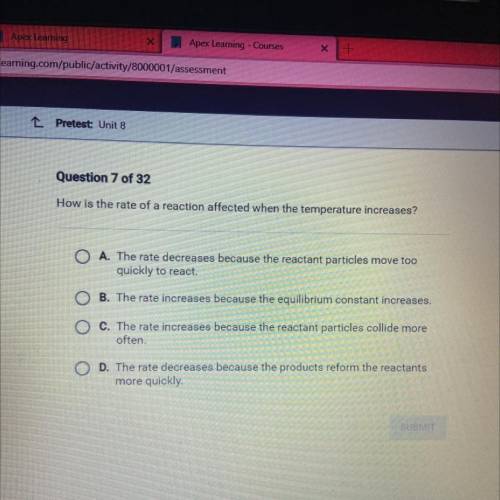
How is the rate of a reaction affected when the temperature increases?
A. The rate decreases because the reactant particles move too
quickly to react.
B. The rate increases because the equilibrium constant increases.
C. The rate increases because the reactant particles collide more
often.
D. The rate decreases because the products reform the reactants more quickly
more quickly.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
How is the rate of a reaction affected when the temperature increases?
A. The rate decreases becaus...
Questions


German, 17.10.2019 19:00

Mathematics, 17.10.2019 19:00

Physics, 17.10.2019 19:00

German, 17.10.2019 19:00

Mathematics, 17.10.2019 19:00




Social Studies, 17.10.2019 19:00






Mathematics, 17.10.2019 19:00




History, 17.10.2019 19:00



