
Chemistry, 16.07.2021 23:30 Mayjay2827
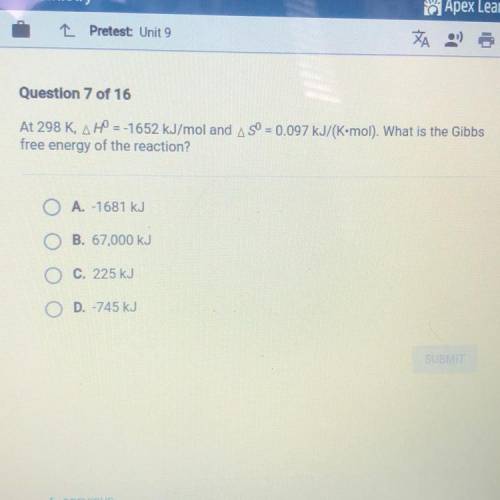
At 298 K, H = -1652 kJ/mol and S = 0.097 kJ/(K•mol). What is the Gibbs free energy of the reaction?
A.-1681 kJ
B. 67,000 kJ
C. 225 kJ
D. -745 kJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3
You know the right answer?
At 298 K, H = -1652 kJ/mol and S = 0.097 kJ/(K•mol). What is the Gibbs free energy of the reaction?...
Questions


Computers and Technology, 10.04.2020 02:51

Mathematics, 10.04.2020 02:51





Mathematics, 10.04.2020 02:51


Mathematics, 10.04.2020 02:51


English, 10.04.2020 02:51




English, 10.04.2020 02:51

History, 10.04.2020 02:51

Mathematics, 10.04.2020 02:51




