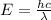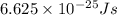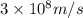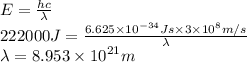5. A beam of photons with a minimum energy of 222 kJ/mol can eject electrons from a potassium surface. Estimate the range of wavelengths of light that can be used to cause this phenomenon. Show your calculations with units of measure (dimensional analysis) and briefly explain your reasoning.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

You know the right answer?
5. A beam of photons with a minimum energy of 222 kJ/mol can eject electrons from a potassium surfac...
Questions






History, 14.02.2020 04:28


Mathematics, 14.02.2020 04:28

Mathematics, 14.02.2020 04:28

Biology, 14.02.2020 04:28

Mathematics, 14.02.2020 04:28





Mathematics, 14.02.2020 04:28




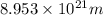 .
.