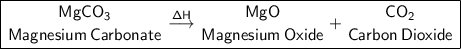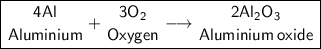Chemistry, 16.07.2021 06:00 rorathan123
Complete and balance the following chemical equations. Identify the reaction type as: combination, decomposition, single replacement, double replacement, or combustion.
Products:
Magnesium Oxide + Carbon dioxide.
a) MgCO₃ (Heat is supplied to the reaction (triangle over a arrow) -> Reaction type:
Products:
Aluminum Oxide
b) Al + O₂ -> Reaction type:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1


Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Complete and balance the following chemical equations. Identify the reaction type as: combination, d...
Questions




Engineering, 16.11.2020 17:50

Mathematics, 16.11.2020 17:50



Mathematics, 16.11.2020 17:50

Mathematics, 16.11.2020 17:50


Mathematics, 16.11.2020 17:50

Medicine, 16.11.2020 17:50


Social Studies, 16.11.2020 17:50

Mathematics, 16.11.2020 17:50



Mathematics, 16.11.2020 17:50

Mathematics, 16.11.2020 17:50

Mathematics, 16.11.2020 17:50