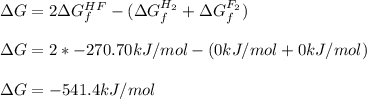Chemistry, 16.07.2021 01:00 theannakittler7327
Hydrogen gas and fluorine gas will react to form hydrogen fluoride gas. What is the standard free energy change for this reaction

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Hydrogen gas and fluorine gas will react to form hydrogen fluoride gas. What is the standard free en...
Questions


Health, 21.05.2021 02:10

Social Studies, 21.05.2021 02:10

Mathematics, 21.05.2021 02:10

Mathematics, 21.05.2021 02:10

Mathematics, 21.05.2021 02:10


Mathematics, 21.05.2021 02:10

Mathematics, 21.05.2021 02:10

Mathematics, 21.05.2021 02:10


Mathematics, 21.05.2021 02:10

Mathematics, 21.05.2021 02:10


Social Studies, 21.05.2021 02:10


Mathematics, 21.05.2021 02:10


English, 21.05.2021 02:10