Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1

Chemistry, 23.06.2019 15:30
An isotope undergoes radioactive decay. the new isotope that forms has an atomic number fhat is 2 less than the original isotopes. which kind of decay has occured and how do you know
Answers: 2
You know the right answer?
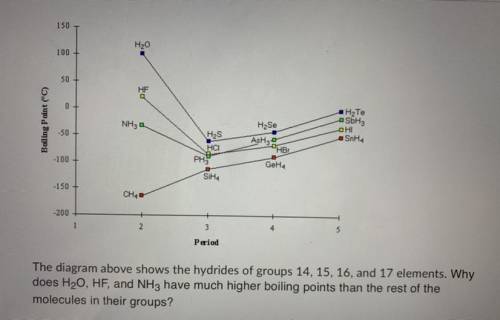
The diagram above shows the hydrides of groups 14, 15, 16, and 17 elements. Why does H20, HF, and NH...
Questions


Biology, 08.10.2021 01:00

Mathematics, 08.10.2021 01:00

Chemistry, 08.10.2021 01:00

Mathematics, 08.10.2021 01:00

Mathematics, 08.10.2021 01:00


Mathematics, 08.10.2021 01:00

Mathematics, 08.10.2021 01:00


Mathematics, 08.10.2021 01:00

Mathematics, 08.10.2021 01:00

Mathematics, 08.10.2021 01:00

Computers and Technology, 08.10.2021 01:00


Mathematics, 08.10.2021 01:00



Mathematics, 08.10.2021 01:00