
Chemistry, 15.07.2021 07:10 xonyemaa12
The equilibrium concentrations for the reaction between SO2 and O2 to form SO3 at a certain temperature are given in the table below. Determine the equilibrium constant and whether the reaction favors reactants, products, or neither at this temperature.
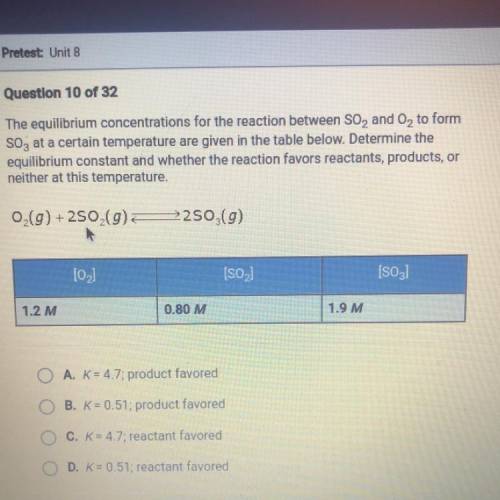

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3


You know the right answer?
The equilibrium concentrations for the reaction between SO2 and O2 to form SO3 at a certain temperat...
Questions


Biology, 13.10.2020 03:01





Computers and Technology, 13.10.2020 03:01

Mathematics, 13.10.2020 03:01

Mathematics, 13.10.2020 03:01


Computers and Technology, 13.10.2020 03:01











