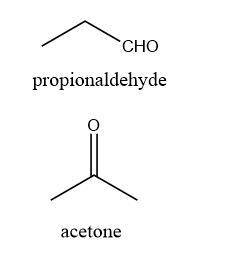
A molecule of acetone and a molecule of propyl aldehyde are both made from 3 carbon atoms, 6 hydrogen atoms, and 1 oxygen atom. The molecules differ in their arrangement of atoms. How do formulas for the two compounds compare? Both compounds have the same molecular formula, but have unique structural formulas. Both compounds have unique molecular formulas and structural formulas. Both compounds have the same structural formula, but have unique molecular formulas.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
A molecule of acetone and a molecule of propyl aldehyde are both made from 3 carbon atoms, 6 hydroge...
Questions




English, 16.04.2021 20:30

Mathematics, 16.04.2021 20:30

Mathematics, 16.04.2021 20:30




Mathematics, 16.04.2021 20:30



Mathematics, 16.04.2021 20:30






Mathematics, 16.04.2021 20:30