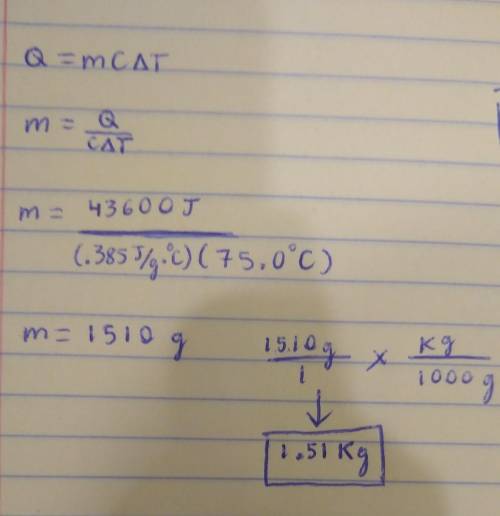Chemistry, 13.07.2021 17:00 darkghostmist
A sample of Copper absorbs 43.6 KJ of heat, resulting in a temperature rise of 75.0 oC, determine the mass (in Kg) of the copper sample, if the specific heat capacity of Copper is 0.385 J/g oC

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
A sample of Copper absorbs 43.6 KJ of heat, resulting in a temperature rise of 75.0 oC, determine th...
Questions



English, 27.04.2020 01:37

History, 27.04.2020 01:37

Mathematics, 27.04.2020 01:37


Social Studies, 27.04.2020 01:37


Mathematics, 27.04.2020 01:37







Mathematics, 27.04.2020 01:37

Arts, 27.04.2020 01:37


Mathematics, 27.04.2020 01:37