
Octane (C8H18) is a component of gasoline that burns according to the following equation: C8H18(l) + 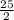 O2(g) → 8CO2(g) + 9H2O(g) ΔH∘rxn = −5074.1 kJ
What mass of octane (in g) is required to produce 8210 kJ of heat?
O2(g) → 8CO2(g) + 9H2O(g) ΔH∘rxn = −5074.1 kJ
What mass of octane (in g) is required to produce 8210 kJ of heat?
Express your answer to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2


Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Octane (C8H18) is a component of gasoline that burns according to the following equation: C8H18(l) +...
Questions

Health, 04.11.2020 20:50

Mathematics, 04.11.2020 20:50

Spanish, 04.11.2020 20:50



English, 04.11.2020 20:50



Mathematics, 04.11.2020 20:50



English, 04.11.2020 20:50




Social Studies, 04.11.2020 20:50

Mathematics, 04.11.2020 20:50


History, 04.11.2020 20:50




