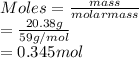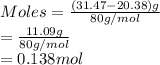Chemistry, 12.07.2021 19:40 Annabel9554
A 20.38 gram sample of cobalt is heated in the presence of excess sulfur. A metal sulfide is formed with a mass of 31.47 g. Determine the empirical formula of the metal sulfide.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
A 20.38 gram sample of cobalt is heated in the presence of excess sulfur. A metal sulfide is formed...
Questions

History, 09.03.2020 05:47

Mathematics, 09.03.2020 05:48

Mathematics, 09.03.2020 05:48

Social Studies, 09.03.2020 05:48

English, 09.03.2020 05:48

Mathematics, 09.03.2020 05:48

Mathematics, 09.03.2020 05:48


Biology, 09.03.2020 05:48

Health, 09.03.2020 05:48

Mathematics, 09.03.2020 05:48

History, 09.03.2020 05:48


Biology, 09.03.2020 05:48


History, 09.03.2020 05:49

Mathematics, 09.03.2020 05:49

Mathematics, 09.03.2020 05:49

Mathematics, 09.03.2020 05:49

 .
.