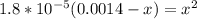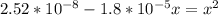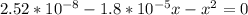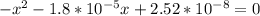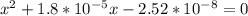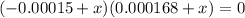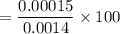Chemistry, 10.07.2021 03:00 ayoismeisjjjjuan
A student prepares a aqueous solution of acetic acid . Calculate the fraction of acetic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
A student prepares a aqueous solution of acetic acid . Calculate the fraction of acetic acid that is...
Questions




Mathematics, 22.06.2019 18:00

History, 22.06.2019 18:00


Mathematics, 22.06.2019 18:00


Mathematics, 22.06.2019 18:00

Social Studies, 22.06.2019 18:00

Mathematics, 22.06.2019 18:00







History, 22.06.2019 18:00


![K_a = \dfrac{[x][x]]}{[0.0014-x]}](/tpl/images/1391/9794/ad446.png)
![1.8*10^{-5} = \dfrac{[x][x]]}{[0.0014-x]}](/tpl/images/1391/9794/49413.png)
![1.8*10^{-5} = \dfrac{[x]^2}{[0.0014-x]}](/tpl/images/1391/9794/caa58.png)