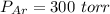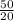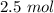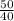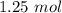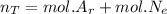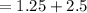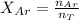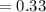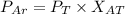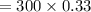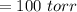Chemistry, 10.07.2021 01:00 kingofmortals2119
A gas mixture, with a total pressure of 300. torr, consists of equal masses of Ne (atomic weight 20.) and Ar (atomic weight 40.). What is the partial pressure of Ar, in torr

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases.as altitude increases, air density increases.air pressure and density are lowest at sea level.denser air exerts more pressure than less dense air.
Answers: 2


Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
A gas mixture, with a total pressure of 300. torr, consists of equal masses of Ne (atomic weight 20....
Questions



English, 19.01.2020 00:31


Social Studies, 19.01.2020 00:31




Mathematics, 19.01.2020 00:31







English, 19.01.2020 00:31

Mathematics, 19.01.2020 00:31

Mathematics, 19.01.2020 00:31