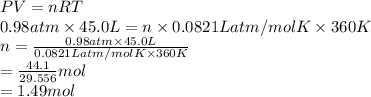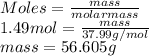Chemistry, 09.07.2021 20:20 kaygirlnelson6258
Answer the following question:
Calculate the mass of 45.0 L of F2 at 87.0° C and 750. mm Hg.
Use the Ideal Gas Law formula and here are values for R:
8.134 (L * kPa)/(mol * K)
0.0821 (atm * L)/(mol * K)
62.364 (L * mmHg)/(mol * K)
Include the following with your
Which Gas Law constant did you use or which "R" value did you use? Why?
The numerical answer to the question.
An explanation of the correct number of significant figures you will use for the numerical answer.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
You know the right answer?
Answer the following question:
Calculate the mass of 45.0 L of F2 at 87.0° C and 750. mm Hg.
<...
<...
Questions

Mathematics, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00


Spanish, 11.11.2020 01:00


Mathematics, 11.11.2020 01:00

Computers and Technology, 11.11.2020 01:00

Geography, 11.11.2020 01:00


Mathematics, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00




Social Studies, 11.11.2020 01:00



Mathematics, 11.11.2020 01:00


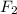 at 87.0° C and 750 mm Hg is 56.605 g.
at 87.0° C and 750 mm Hg is 56.605 g. 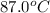 = (87.0 + 273) K = 360 K
= (87.0 + 273) K = 360 K