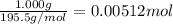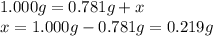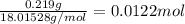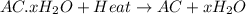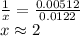A sample of unknown hydrate, AC-XH20, has a mass of 1.000 g before heating and a
mass of 0.781 g after heating. If the molar mass of the anhydrous compound (AC) is
195.5 g/mol, what is the water of crystallization for the formula of the unknown
hydrate?
Type your work for partial credit.
Answer choices: 2, 3, 5, or 6.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

You know the right answer?
A sample of unknown hydrate, AC-XH20, has a mass of 1.000 g before heating and a
mass of 0.781 g af...
Questions


Mathematics, 23.01.2021 19:30


Mathematics, 23.01.2021 19:30


Geography, 23.01.2021 19:30



Spanish, 23.01.2021 19:30



Physics, 23.01.2021 19:30



Mathematics, 23.01.2021 19:30

English, 23.01.2021 19:30

Mathematics, 23.01.2021 19:30


Physics, 23.01.2021 19:30