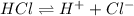Chemistry, 07.07.2021 01:00 laytonlutz
A Bronsted-Lowry acid is defined as a substance that . A Bronsted-Lowry acid is defined as a substance that . increases Ka when placed in H2O increases [OH-] when placed in H2O acts as a proton donor acts as a proton acceptor decreases [H ] when placed in H2O

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
A Bronsted-Lowry acid is defined as a substance that . A Bronsted-Lowry acid is defined as a substan...
Questions


Mathematics, 09.02.2021 19:10



Mathematics, 09.02.2021 19:10


Mathematics, 09.02.2021 19:10

English, 09.02.2021 19:10


Mathematics, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10

History, 09.02.2021 19:10



Mathematics, 09.02.2021 19:10


Mathematics, 09.02.2021 19:10