Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
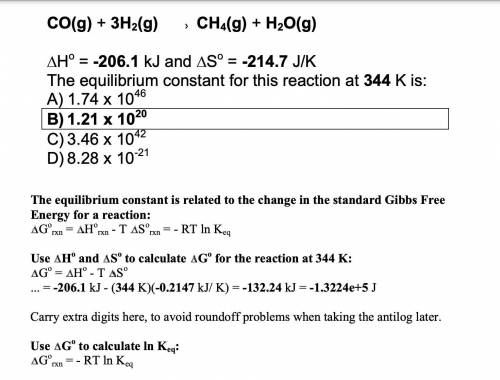
Calculate the numerical Kc value for the following reaction if the equilibrium mixture contains 0.51...
Questions

History, 27.05.2021 23:30

History, 27.05.2021 23:30

Mathematics, 27.05.2021 23:30

Mathematics, 27.05.2021 23:30

History, 27.05.2021 23:30








Social Studies, 27.05.2021 23:30

Mathematics, 27.05.2021 23:30



Chemistry, 27.05.2021 23:30