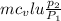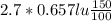Chemistry, 02.07.2021 22:50 makenahbriana
A 1.5-m 3 insulated rigid tank contains 2.7 kg of carbon dioxide at 100 kPa. Now paddle-wheel work is done on the system until the pressure in the tank rises to 150 kPa. Determine the entropy change of carbon dioxide during this process. Assume constant specific heats

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2
You know the right answer?
A 1.5-m 3 insulated rigid tank contains 2.7 kg of carbon dioxide at 100 kPa. Now paddle-wheel work i...
Questions



Chemistry, 02.11.2019 23:31



Mathematics, 02.11.2019 23:31


History, 02.11.2019 23:31


Chemistry, 02.11.2019 23:31


Mathematics, 02.11.2019 23:31



Advanced Placement (AP), 02.11.2019 23:31


Mathematics, 02.11.2019 23:31

English, 02.11.2019 23:31