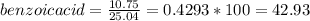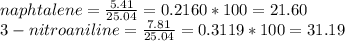Chemistry, 02.07.2021 04:40 lydiapoetz5330
1. Calculate the percent recovery of benzoic acid, naphthalene and 3-nitroaniline if you were able to collect 10.75 g of benzoic acid, 5.41 g of naphthalene, and 7.81 g of 3-nitroaniline from a set of extractions. The starting mass of the mixture was 25.04 g. (0.6 pt) 2. Describe why it is important to use sodium hydroxide and hydrochloride acid in this experiment. Why was it necessary to initially start off with a 5% solution of the acid or base for this experiment

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

You know the right answer?
1. Calculate the percent recovery of benzoic acid, naphthalene and 3-nitroaniline if you were able t...
Questions


Biology, 14.04.2020 18:50

Mathematics, 14.04.2020 18:50

Mathematics, 14.04.2020 18:50

Mathematics, 14.04.2020 18:50

Mathematics, 14.04.2020 18:50

Chemistry, 14.04.2020 18:50

English, 14.04.2020 18:50












Health, 14.04.2020 18:50