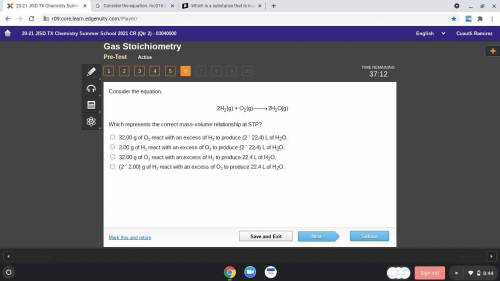
Consider the equation.
Which represents the correct mass-volume relationship at STP?
32.00 g...

Chemistry, 01.07.2021 17:00 juliannabartra
Consider the equation.
Which represents the correct mass-volume relationship at STP?
32.00 g of O2 react with an excess of H2 to produce (2 ´ 22.4) L of H2O.
2.00 g of H2 react with an excess of O2 to produce (2 ´ 22.4) L of H2O.
32.00 g of O2 react with an excess of H2 to produce 22.4 L of H2O.
(2 ´ 2.00) g of H2 react with an excess of O2 to produce 22.4 L of H2O.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3
You know the right answer?
Questions







Mathematics, 22.10.2019 16:50


Mathematics, 22.10.2019 16:50






Biology, 22.10.2019 16:50



History, 22.10.2019 16:50

Physics, 22.10.2019 16:50



