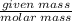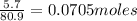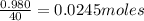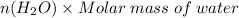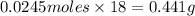Aqueous hydrobromic acid (HBr) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H2O). Suppose 5.7 g of hydrobromic acid is mixed with 0.980 g of sodium hydroxide. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1

Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1
You know the right answer?
Aqueous hydrobromic acid (HBr) will react with solid sodium hydroxide (NaOH) to produce aqueous sodi...
Questions

Mathematics, 30.07.2021 21:50

Mathematics, 30.07.2021 21:50



Mathematics, 30.07.2021 21:50


Mathematics, 30.07.2021 22:00

History, 30.07.2021 22:00

Mathematics, 30.07.2021 22:00

Biology, 30.07.2021 22:00

Health, 30.07.2021 22:00




Biology, 30.07.2021 22:00

Mathematics, 30.07.2021 22:00

Mathematics, 30.07.2021 22:00


History, 30.07.2021 22:00

Social Studies, 30.07.2021 22:00